Preimplantation Genetic Diagnosis (PGD) of embryos can avoid serious hereditary diseases

Genetic Screening and Diagnosis
The Fertility Institutes have been providing advanced genetic analysis of embryos to avoid serious genetic diseases for well over two decades. As one of the worlds largest providers of Preimplantation Genetic Diagnosis (PGD) genetic screening to the International community, the physicians and scientists at the Fertility Institutes have unmatched experience in coordinating, accommodating and facilitating the complex medical procedures involved in advanced genetic screening of human embryos. You could not choose a more dedicated, experienced group of physicians and scientists than those at the Fertility Institutes.

Once you contact us, you will receive detailed instructions for providing our Doctors with the important details of your or your family's history with sickle cell disorder. The Doctors will request additional preliminary testing and you will be sent special kits to have your blood collected and shipped to our laboratories in the United States. In most instances, arrangements for treatment can be carried out within 30-60 days of your initial contact with us.
Among the Diseases Detectable with PGD
The accuracy of PGD in determining genetic abnormalities exceeds 98%. Once we have the genetic information about each embryo available, we are able to sit down with each couple prior to the embryo transfer, discuss the genetic health of each embryo, explain how the genetic information has improved the chances for pregnancy success compared to their prior unsuccessful attempts at IVF elsewhere, and make a determination about the return of the now "known to be normal" embryos to the mother to be. Because PGD so improves the success of IVF, many couples elect, in consultation with us to decrease the total number of embryos placed back in the womb. This also decreases the chance of multiple birth and the chance of prematurity. It also allows for the cryopreservation of extra "normal" embryos for future pregnancies. Our experience has shown that even when far fewer embryos are returned to the uterus, pregnancy success increases following PGD.
Current Detectable Diseases
- Adrenoleukodystrophy
- Amyotrophic Lateral Sclerosis
- Becker Muscular Dystrophy
- Beta Thalassemia
- BREAST CANCER
- Central Core Disease
- Centronuclear (Myotubular) Myopathy
- Cerebellar Ataxia
- Charcot-Marie-Tooth Disease
- Chondrodysplasia Punctata
- Congenital Aganglionic Megacolon
- Conradi-Hunnerman Syndrome
- Cystic Fibrosis
- Duchenne Muscular Dystrophy
- Factor VIII Deficiency
- Factor IX Deficiency
- Familial Spastic Paraparesis
- Fragile X Syndrome
- Friedrich's Ataxia
- Gardener Syndrome
- Glycogen Storage Disease
- Happle Syndrome
- Hemophilia
- Huntington's Disease
- Retinitis Pigmentosa
- Prostate Cancer
- Sickle Cell Anemia
- Tay-Sachs Disease
- Von Willebrand Disease
- Many Other Diseases
See an example of a PGD genetic analysis report. Though these embryos appeared "normal" under the microscope, it can be seen that many carried genetic abnormalities that would not allow for pregnancy. Only those truly "normal" embryos were returned to the mother, with a healthy twin pregnancy resulting.
PGD Technology Can Guarantee a Sickle Cell Free Baby
Sickle cell anemia is an inherited disease of the red blood cells which can cause attacks of pain and damage to vital organs which can lead to premature death. The genetic basis of sickle cell inheritance has been known for many years. It is just recently however that this genetic knowledge has become useful in helping those families known to carry the sickle cell gene avoid the birth of infants affected with the sickle cell disease disorder. The sickle cell prevention PGD program at the Fertility Institutes in Los Angeles and New York approaches 100% certainty in helping couples assure that a pregnancy will not result in the birth of a baby with sickle cell disease.

The sickle cell disorder is known to occur when a person inherits two sickle cell genes (one from each parent) or a combination of one sickle cell gene from one parent and any one of several other abnormal hemoglobin genes from the other. The sickle cell disorder may be the world's most commonly inherited disorder and is clearly the most common genetic affliction seen in African infants. 1% to 2% of babies born on the African continent are afflicted with sickle cell disorder or one of its variants.
The genes for the sickle related disorders arose in the evolution of mankind as a result of gene mutation. Because of the partial protective effects of sickle genes against the dangers of falciparium malaria, these gene abnormalities proliferated in areas where there was or is a high incidence of malaria. Currently, the Bantus in African countries north of the Zambesi River have a high incidence of sickle cell disorder, with the prevalence varying but significant in all other parts of the African continent.
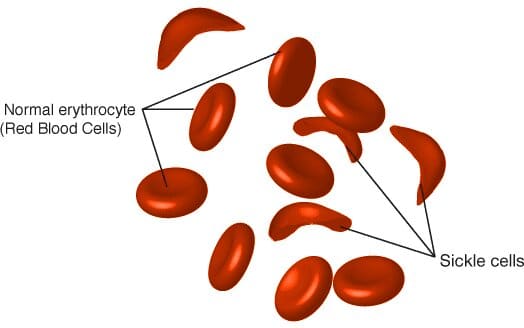
The inheritance of a sickle cell related disorder occurs at the moment of conception. As a human egg carrying a sickle trait is initially fertilized by a sperm carrying a similar disorder, the resulting new embryo is irreversibly imprinted with the genetic blueprint that will ultimately and inevitably appear as a sickle cell related disorder in the newborn.
This early imprinting of the embryo allows the physicians and scientists at the Fertility Institutes the opportunity to analyze the newly formed embryos in search of the genetic codes responsible for both normal and abnormal hemoglobin production, sickle cell and non sickle cell embryos. Prior to this analysis, the future mother and father will have had blood studies carried out by the Fertility Institutes to identify and characterize the nature of the sickle related genetic disorder that is carried. This information is then used to aid in the identification of both normal embryos resulting from the new fertilization as well as those embryos destined to exhibit a sickle cell related disorder. The embryos being examined remain in the safe confines of the embryo growth and development incubators at the world renown Fertility Institutes. Within a few short days of the production of the embryos, the genetic teams associated with the Fertility Institutes will have studied the embryos and identified those both disease free as well as those carrying the sickle cell related disorder. This vital genetic information is then shared by the physicians with the parents allowing parents the opportunity to avoid with near certainty the chance of a pregnancy carrying a predefined sickle related disorder.
Entering the Sickle Cell Screening International Program
Those interested in utilizing the services of the Fertility Institutes may begin by simply contacting our offices. From Nigeria, the phone number is 009 1 (818) 728-4600. From other nations on the African continent, the International dialing code for the United States should be used followed by our 7 digit direct line of (818) 728-4600. For an immediate conversation with one of our International Coordinators, please call from 9AM to 4PM Pacific time. Calls at other times will be answered and a request for a return contact telephone number will be taken and you will be called the next business day.
In vitro fertilization allows for Preimplantation Genetic Diagnosis and Screening
In vitro fertilization offers a unique opportunity to test the growing embryo for genetic problems. To understand the process of preimplantation genetic diagnosis one must first understand the process of in vitro fertilization. In vitro fertilization involves the collection of the female half of a new pregnancy to be, the egg, and the male half, the sperm, and the subsequent uniting of them in a very specialized and secure laboratory devoted to this delicate process.
The eggs are collected surgically after ovarian stimulation and the triggering of ovulation. Simultaneous to the egg collection, sperm are isolated from a freshly acquired semen sample. An egg and a single isolated sperm are then united in a process called intracytoplasmic sperm injection (ICSI). In this procedure a healthy appearing sperm is inserted into the egg. The ICSI procedure is required for preimplantation genetic diagnosis because the genetic testing is so sensitive that any extra sperm present near the egg may confound the results of the highly sensitive genetic tests to be performed on the pregnancy to be. Despite physical union of the sperm and the egg at this point, the genetic union cannot be detected until the next day.
On the first day after the egg retrieval and ICSI procedure, the new unique embryo can first be detected. Embryos at this point have two nuclei, a male pronucleus and a female pronucleus, and the embryo is at the 2 pronuclear (2PN) stage. These newly created embryos are subsequently grown under 24 hour monitoring for 5-6 days, progressing from cleavage (growing) stage embryos on the third day (4 to 10 cell stage) to the blastocyst stage (growing and differentiating, with both a fetal side and a placental side). Embryo biopsy, or the genetic testing of the embryo can be done at all stages, whether from the egg itself through polar body biopsy, or at the cleavage stage where one cell may be removed from the embryo without detriment, or at the blastocyst stage where 10-12 cells may be removed without damaging the growing embryo. Please see below figure for a diagram of the different stages including the egg (polar body biopsy), the cleavage embryo (Day 3 biopsy), and the differentiating blastocyst (Day 5 biopsy). Owing to improved accuracy and survivability, we uniformly perform biopsies at the blastocyst stage.
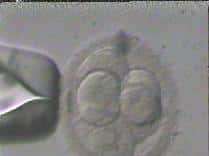
1. A 7 cell embryo prepared for biopsy on culture day 3
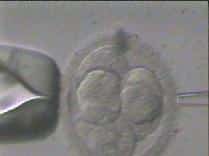
2. A gentle acid solution is applied to the zona "shell" of the embryo, allowing access to the blastomeres contained within

3. The zona "shell" has been penetrated and opened. A single cell is selected for biopsy
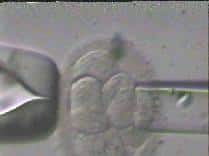
4. The selected cell is gently separated from it's neighbor cells and drawn into the biopsy instrument
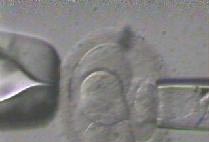
5. The blastomere is removed. Genetic information is contained within the small dimple seen within each cell

6. Separation from the cell is complete and the biopsy probe with the contained cell is drawn away from the embryo
PGD and Amniocentesis
PGD cannot substitute for mid-trimester genetic ultrasound evaluation and amniocentesis or chorionic villi sampling. We encourage additional genetic testing once a successful pregnancy has been established to confirm the total genetic health of the resulting pregnancy.
Financial Considerations
Total costs for PGD genetic screening for sickle cell disorders will vary slightly depending upon the nature of the genetics being studied. Average costs for the medical and genetic portions of the service provided by the Fertility Institutes approach $27,000 U.S. Travel, lodging and the medical fees to the local African center assisting in your preparation will be extra. All services are provided at both our Los Angeles and New York locations.
If you or a family member are living with a sickle cell related disorder and you have an interest in assuring a non-affected pregnancy, we invite you to contact us by phone, by email at Sickle Cell Inquiry or by filling out the following contact inquiry form. You will be contacted shortly by one of our experienced staff members. Thank you for you interest in the Sickle Related Disorders PGD Screening Program at the Fertility Institutes.

